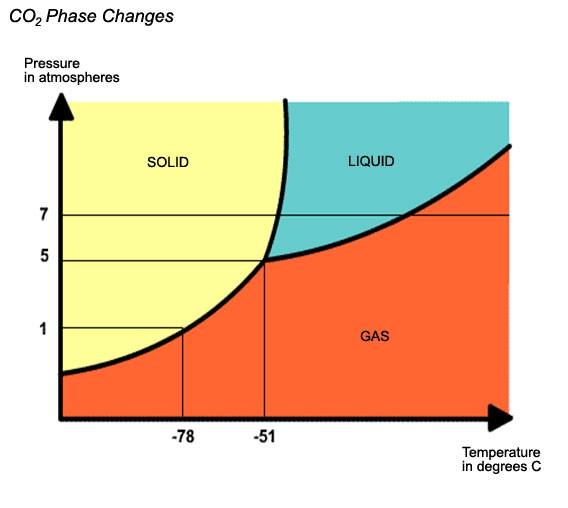
- Dry ice is produced by compressing the CO2 while it is in its liquid form.
- From the liquid CO2, the heat produced by the compression is removed. Then it is solidified by leaving the liquid carbon dioxide to expand rapidly.
- This expansion causes a drop in temperature resulting in part from the CO2 to freeze into snow form, which is then compressed in either crushed (pellets) or in pieces (slices or blocks).
- When some of the CO2 is lost during the sublimation to the atmosphere, no more CO is produced2 and therefore it does not contribute to the greenhouse effect. Instead, only the original CO2 is lost and returned to the atmosphere. That is why the dry ice is considered a carbon neutral product that’s also environmentally friendly.
Carbon dioxide (chemical formula = CO2 ) is a natural by-product of respiration, fermentation and other industrial processes. Carbon is a colorless, odorless and non-flammable gas. Its density at 25 ° C is 1,98 kg / m3, i.e. approximately 1.65 time denser than air. Thus, CO2 gas displaces oxygen in the environment.
Dry ice is the solid form of Carbon Dioxide at a temperature of - 78.5 C. and is white and opaque in appearance. Handling and storage of CO2, in solid or gaseous form, demands well ventilated spaces as a prerequisite.
Dry ice can sublimate or change directly from solid to gas form without the liquid phase. The sublimation speed must be considered when filling a system reservoir, as dry ice decreases in size. Dry ice sublimates by 3% to 8% of its volume on the day, depending on the thermal properties of the reservoir and the outside temperature. A pound of dry ice sublimates to 8.3 cubic feet of carbon dioxide gas.